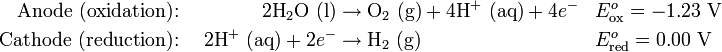|
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water. This electrolytic process is used in some industrial applications when hydrogen is needed.
An electrical power source is connected to two electrodes, or two plates, (typically made from some inert metal such as platinum or stainless steel) which are placed in the water. Hydrogen will appear at the cathode (the negatively charged electrode, where electrons are pumped into the water), and oxygen will appear at the anode (the positively charged electrode). The generated amount of hydrogen is twice the amount of oxygen, and both are proportional to the total electrical charge that was sent through the water.
Electrolysis of pure water is very slow, and can only occur due to the self-ionization of water. Pure water has an electrical conductivity about one millionth that of seawater. It is sped up dramatically by adding an electrolyte (such as a salt, an acid or a base).
Historically, the first known electrolysis of water was done by William Nicholson and Anthony Carlisle in about 1800.
Thermodynamics of the process
Decomposition of pure water into hydrogen and oxygen at standard temperature and pressure is not favorable in thermodynamical terms. This is because, E(cell)=E(Oxidation) + E(Reduction). If E(cell) < 0, reaction is not favorable.
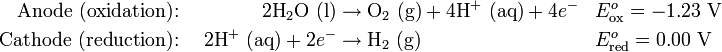
Thus, the standard potential of the water electrolysis cell is 1.23 V at 25 °C.
The positive voltage indicates the Gibbs Free Energy for electrolysis of water is greater than zero for these reactions. This can be found using the Nernst Equation
at equilibrium. The reaction cannot occur without adding necessary
energy, usually supplied by an external electrical power source.
Electrolyte selection
If the above described processes occur in pure water, H+ cations will accumulate at the anode and OH− anions will accumulate at the cathode. This can be verified by adding a pH indicator
to the water: the water near the anode is acidic while the water near
the cathode is basic. These charged ions will repel the further flow of
electricity until they have diffused away, a slow process. This is why pure water conducts electricity poorly and why electrolysis of pure water proceeds slowly.
If a water-soluble electrolyte is added, the conductivity of the water rises considerably. The electrolyte disassociates into cations and anions; the anions rush towards the anode and neutralize the buildup of positively charged H+ there; similarly, the cations rush towards the cathode and neutralize the buildup of negatively charged OH− there. This allows the continued flow of electricity.
Care must be taken in choosing an electrolyte, since an anion from the electrolyte is in competition with the hydroxide ions to give up an electron. An electrolyte anion with less standard electrode potential than hydroxide will be oxidized instead of the hydroxide, and no oxygen gas will be produced. A cation with a greater standard electrode potential than a hydrogen ion will be reduced in its stead, and no hydrogen gas will be produced.
The following cations have lower electrode potential than H+ and are therefore suitable for use as electrolyte cations: Li+, Rb+, K+, Cs+, Ba2+, Sr2+, Ca2+, Na+, and Mg2+. Sodium and lithium are frequently used, as they form inexpensive, soluble salts.
If an acid is used as the electrolyte, the cation is H+, and there is no competitor for the H+ created by disassociating water. The most commonly used anion is sulfate (SO42-), as it is very difficult to oxidize, with the standard potential for oxidation of this ion to the peroxodisulfate ion being −0.22 volts.
Strong acids such as sulfuric acid (H2SO4), and strong bases such as potassium hydroxide (KOH), and sodium hydroxide (NaOH) are frequently used as electrolytes.
A solid polymer electrolyte can also be used such as NAFION
and when applied with a special catalyst on each side of the membrane
can efficiently split the water molecule with as little as 1.8 Volts.
Techniques
Fundamental Demonstration
Two leads, running from the terminals of a battery, are placed in a cup of water with a quantity of electrolyte (not NaCl, anode creates chlorine gas) added to establish conductivity. Hydrogen and oxygen gases will stream from the oppositely charged electrode. Oxygen will collect at the anode and hydrogen will collect at the cathode.
Hofmann voltameter
The Hofmann voltameter is often used as a small-scale electrolytic
cell. It consists of three joined upright cylinders. The inner cylinder
is open at the top to allow the addition of water and the electrolyte. A platinum
electrode is placed at the bottom of each of the two side cylinders,
connected to the positive and negative terminals of a source of electricity. When current is run through the hofmann voltameter, gaseous oxygen forms at the anode and gaseous hydrogen at the cathode. Each gas displaces water and collects at the top of the two outer tubes, where it can be drawn off with a stopcock.
Industrial electrolysis
Many industrial electrolysis cells are very similar to Hofmann voltameters,
with complex platinum plates or honeycombs as electrodes. Generally the
only time hydrogen is intentionally produced from electrolysis is for
specific point of use application such as is the case with oxyhydrogen
torches or when extremely high purity hydrogen or oxygen is desired.
The vast majority of hydrogen is produced from hydrocarbons and as a
result contains trace amounts of carbon monoxide among other impurities. The carbon monoxide impurity can be detrimental to various systems including many fuel cells.
High-temperature electrolysis
High-temperature electrolysis (also HTE or steam electrolysis) is a
method currently being investigated for water electrolysis with a heat engine.
High temperature electrolysis is more efficient than traditional
room-temperature electrolysis because some of the energy is supplied as
heat, which is cheaper than electricity, and because the electrolysis
reaction is more efficient at higher temperatures.
Applications
About four percent of hydrogen
gas produced worldwide is created by electrolysis. The majority of this
hydrogen produced through electrolysis is a side product in the
production of chlorine.
2 NaCl + 2 H2O → Cl2 + H2 + 2 NaOH
The electrolysis of brine, a water sodium chloride mixture, is only half the electrolysis of water since the chloride ions are oxidized to chlorine rather than water being oxidized to oxygen.
The hydrogen produced from this process is either burned, used for the
production of specialty chemicals, or various other small scale
applications.
The majority of hydrogen used industrially is derived from fossil
fuels. One example is fossil fuel derived hydrogen used for the
creation of ammonia for fertilizer via the Haber process and for converting heavy petroleum sources to lighter fractions via hydrocracking. The production of this hydrogen usually involves the formation of synthesis gas a mixture of H2 and CO. Synthesis gas can be hydrogen enriched through the water gas shift reaction. In this reaction the carbon monoxide is reacted with water to produce more H2 with CO2 byproduct.
Efficiency
Water electrolysis does not convert 100% of the electrical energy
into the chemical energy of hydrogen. The process requires more extreme
potentials than what would be expected based on the cell's total
reversible reduction potentials. This excess potential accounts for various forms of overpotential by which the extra energy is eventually lost as heat. For a well designed cell the largest overpotential is the reaction overpotential for the four electron oxidation of water to oxygen at the anode. An effective electrocatalyst
to facilitate this reaction has not been developed. Platinum alloys are
the default state of the art for this oxidation. The reverse reaction,
the reduction of oxygen to water, is responsible for the greatest loss
of efficiency in fuel cells. Developing a cheap effective electrocatalyst for this reaction would be a great advance.
The simpler two-electron reaction to produce hydrogen at the cathode can be electrocatalyzed with almost no reaction overpotential by platinum or in theory a hydrogenase enzyme. If other, less effective, materials are used for the cathode then another large overpotential must be paid.
The energy efficiency of water electrolysis varies widely with the numbers cited below on the optimistic side. Some report 50–80%.
These values refer only to the efficiency of converting electrical
energy into hydrogen's chemical energy. The energy lost in generating
the electricity is not included. For instance, when considering a power
plant that converts the heat of nuclear reactions into hydrogen via
electrolysis, the total efficiency may be closer to 30–45%.
References
For More Information: Electrolysis: K-12 Experiments & Background Information
Source: Wikipedia (All text is available under the terms of the Creative Commons Attribution-ShareAlike License)
|